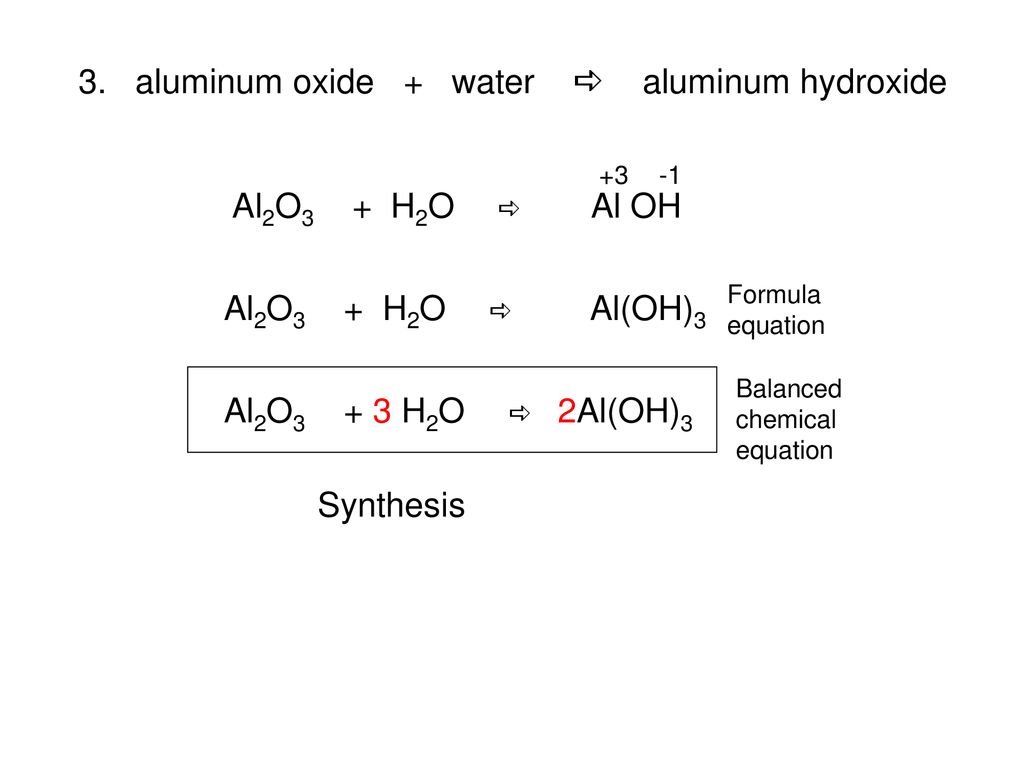
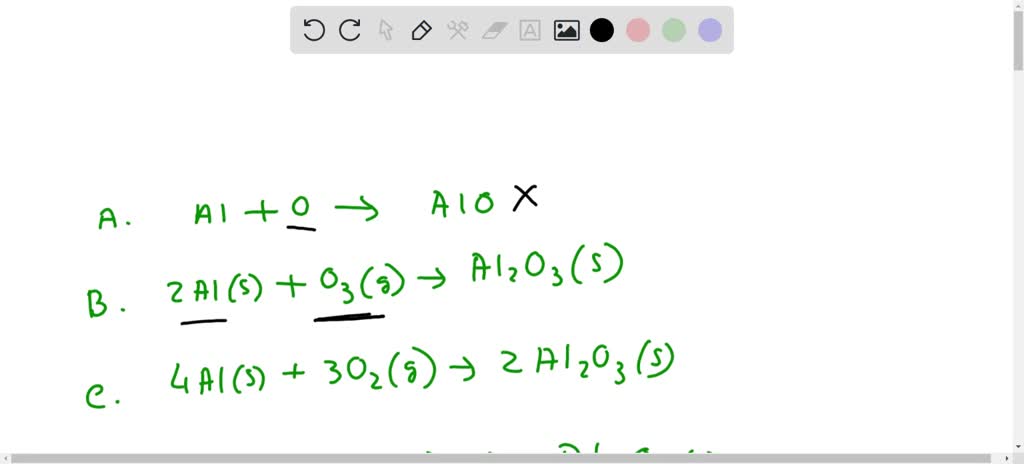
Al-alcl3-al(oh)3-al2o3-и посложнее )) (На картинке ) Составьте уравнения :) Спасибо заранее - Школьные Знания.com
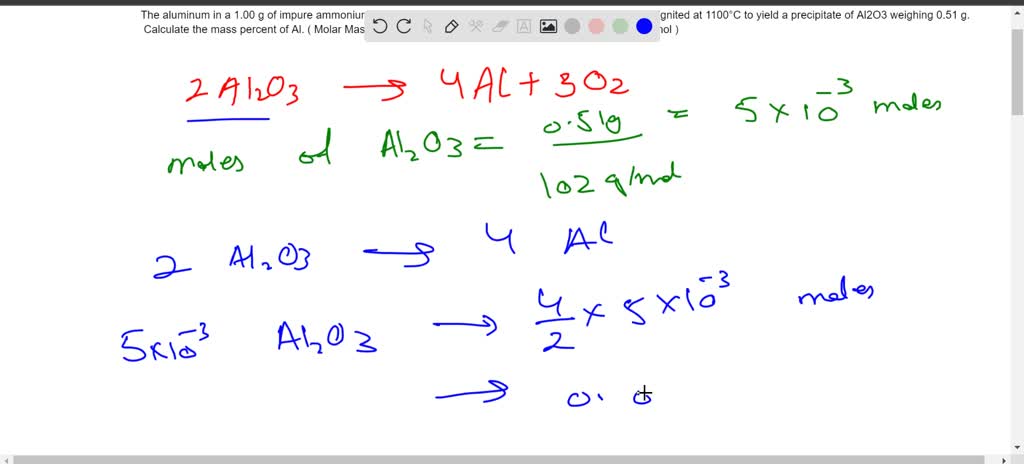
SOLVED: The aluminum in a 1.00 g of impure ammonium aluminum sulfate sample was precipitated as Al(OH)3 and ignited at 1100°C to yield a precipitate of Al2O3 weighing 0.51 g. Calculate the

4/27/16 Today I will define stoichiometry and calculate mole-mole stoichiometry problems Warm Up Write a balanced equation for the reaction of magnesium. - ppt download

Figure 2 from In situ EELS study of dehydration of Al(OH)₃ by electron beam irradiation. | Semantic Scholar

Question: Balance following equations: C3H8 O2 → CO2 H2O Al2(SO3)3 NaOH → Na2SO3 Al(OH)3 Al2O3 - Chem 101 - Stuvia US

0.078 g Al(OH)(3). is dehydrated to Al(2)O(3). The Al(2)O(3) so obtained reacted with 6 milli-equivalent of HCl. The equivalent of AlCl(3) produced during the reaction are: