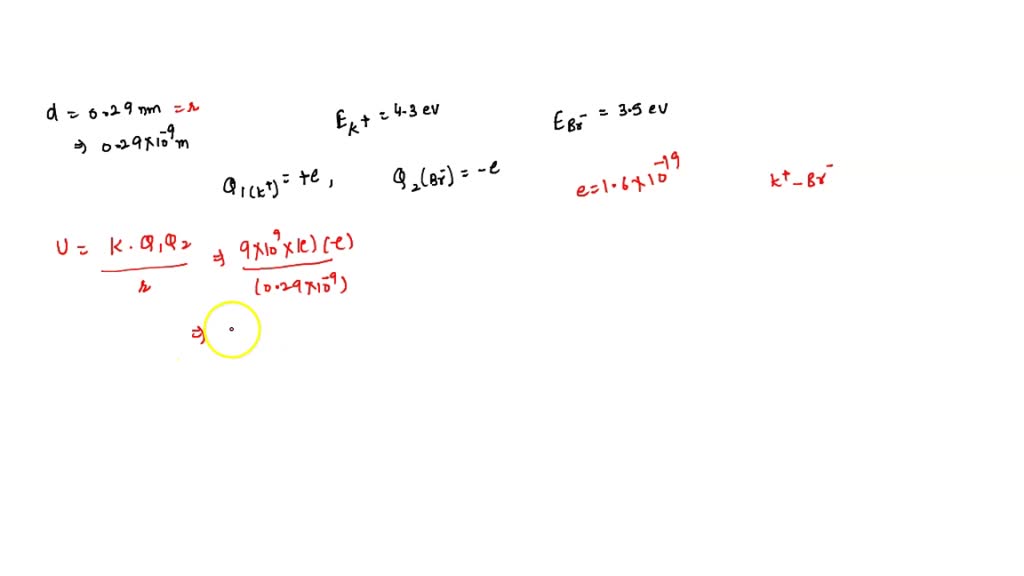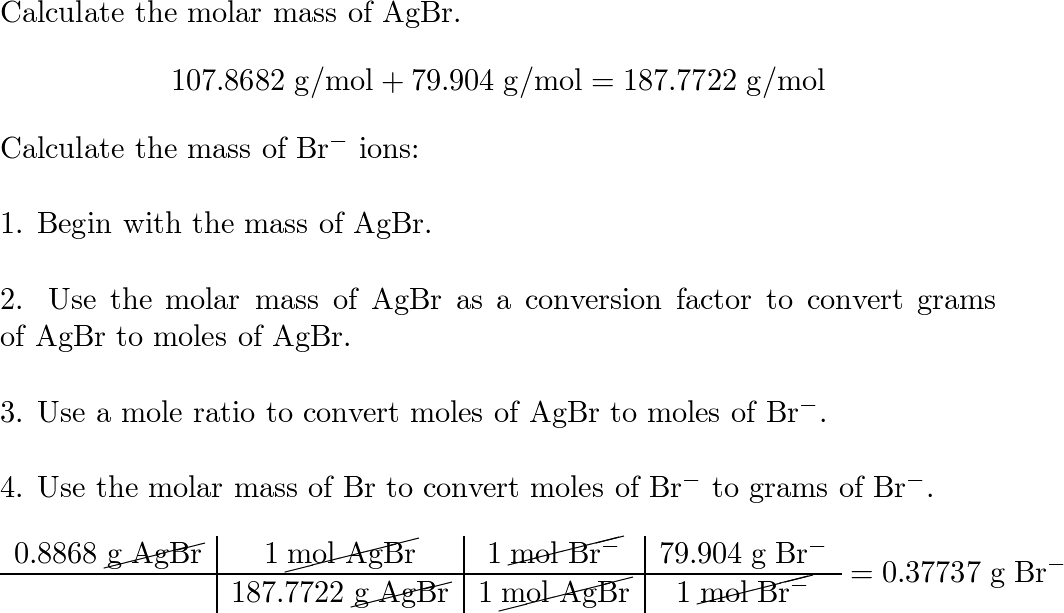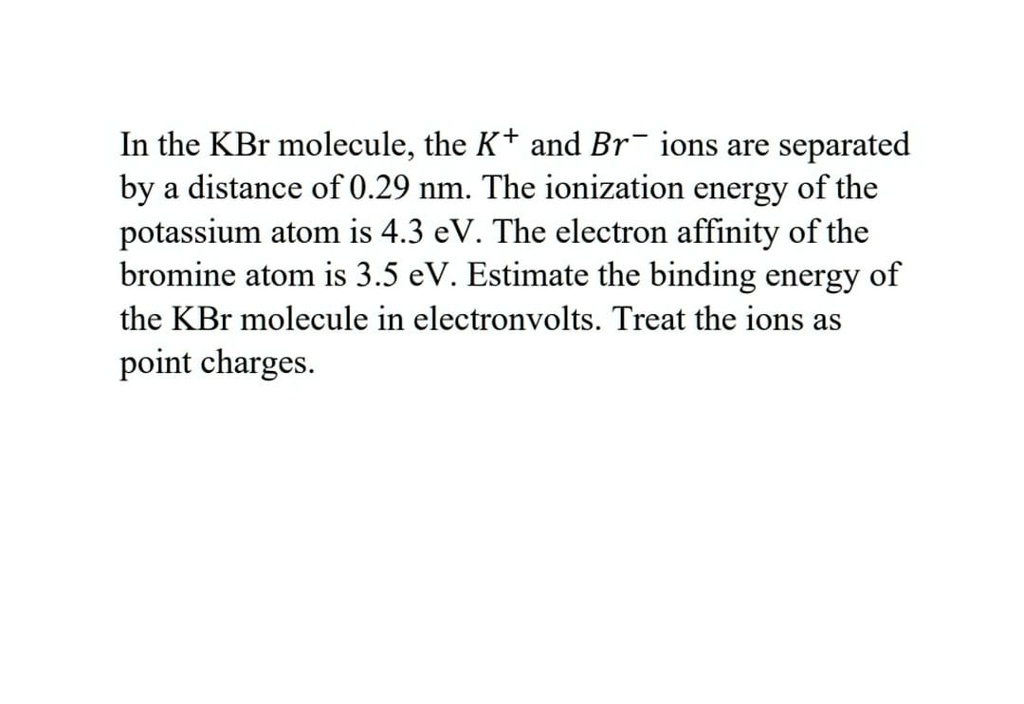
SOLVED: In the KBr molecule, the K+ and Br ions are separated by a distance of 0.29 nm: The ionization energy of the potassium atom is 4.3 eV. The electron affinity of

When BrO3^ - ion reacts with Br^ - ion in acid solution and Br2 is liberated. The equivalent weight of KBrO3 in this reaction is:

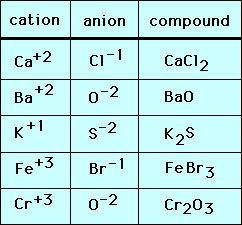
SOLVED: Which of the following atoms would be expected to form negative ions in binary ionic compounds and which would be expected to form positive ions: Br, Ca, Na, N, F, Al,
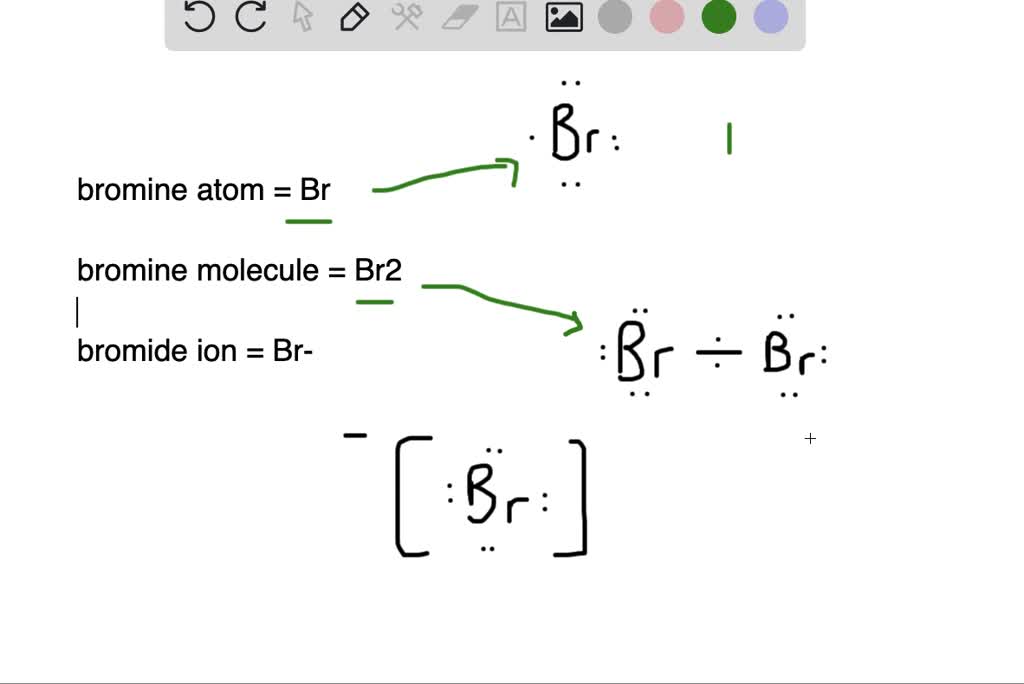
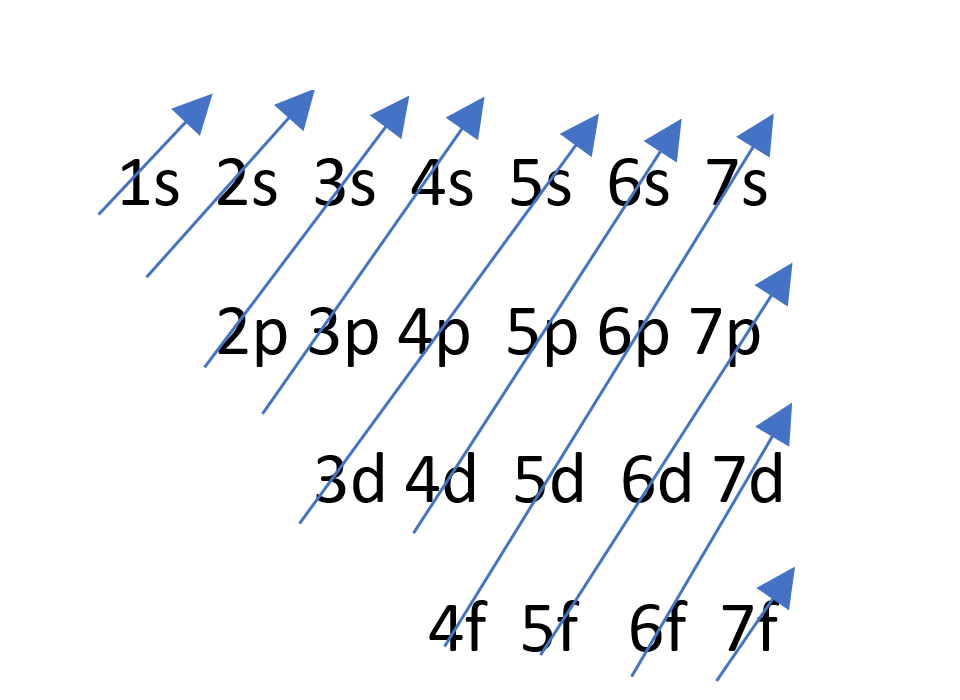
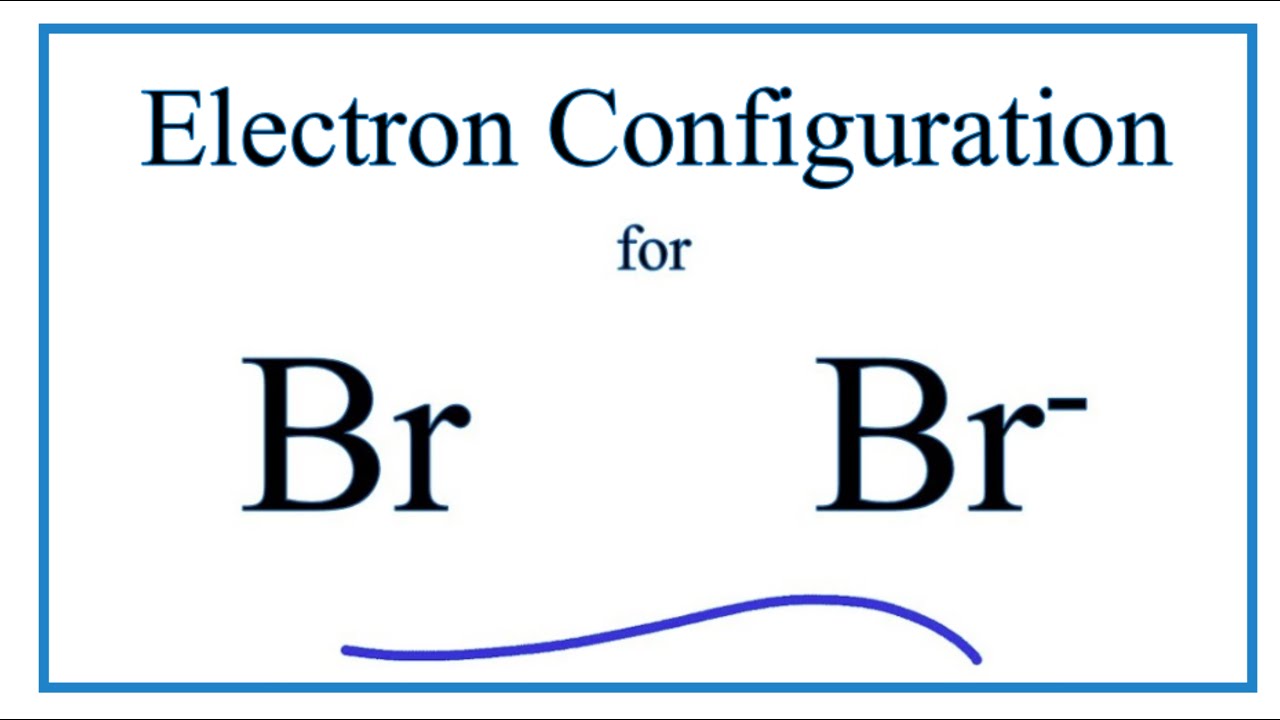
SOLVED: 3 . Explain the difference between bromine atom and bromide ion by writing the configuration of each using the spdf method 4. Write the formula and name of the resulting product

Use Lewis symbols to represent the transfer of electrons between the following atoms to form ions with noble gas configurations: a. Ca and Br. b. K and I. | Homework.Study.com
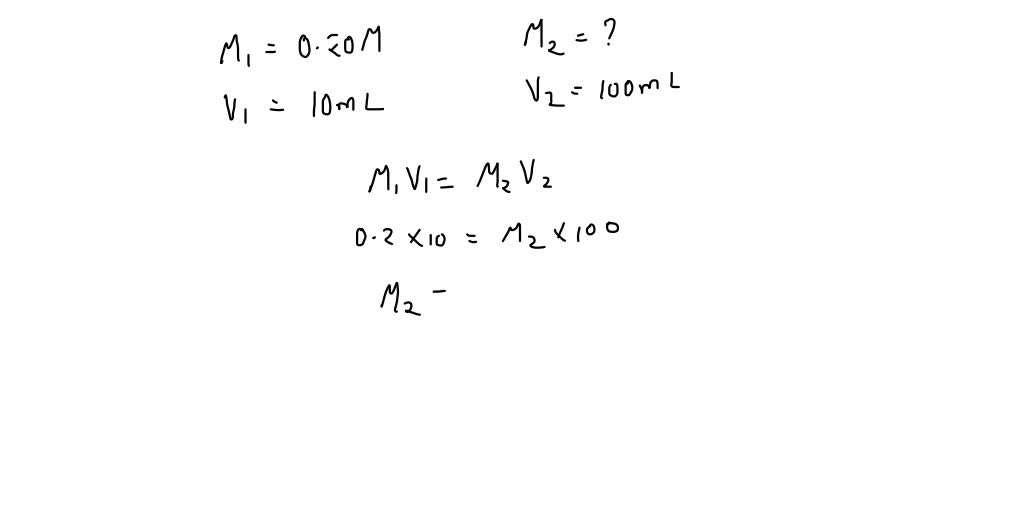
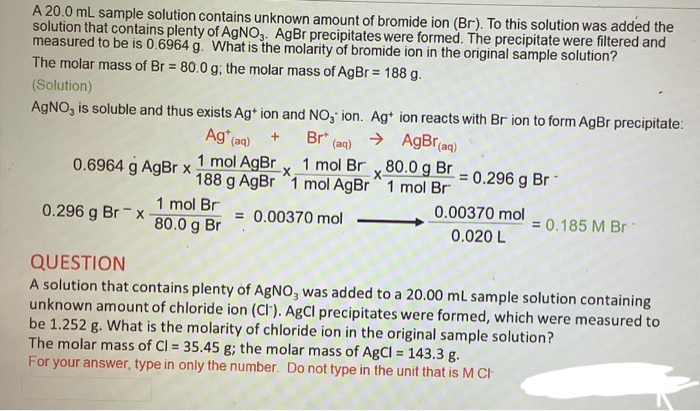
SOLVED: What is the concentration of bromide ion (Br-) in a solution made by diluting 10.00 mL of 0.20 M aluminum bromide (AlBr3) to 100.0 mL?

Br2=BrO3^-+Br^- balance the redox reaction in an acidic medium. br2=bro3^-+ br^- @mydocumentary838 - YouTube